
FLORIDA
Program Overview

Florida
Experience Chemistry
2
DESIGNED TO
ENGAGE STUDENTS
with phenomena-driven instruction using
hands-on and virtual labs, simulations, and
multimedia resources from a variety of sources,
including our partner Flinn Scientific
®
.
DESIGNED FOR FLORIDA
STANDARDS MASTERY
with rigorous, technology-enhanced questions
that align 100% with Florida Standards and
include no extraneous content.
DESIGNED TO
SUPPORT TEACHERS
with comprehensive resources, online tools,
and targeted strategies to support students
and save time.
Immersive Learning Experiences for
Florida Classrooms

Experience the
POSSIBLE
3
With Florida Experience Chemistry, it IS possible to meet your
Florida Chemistry standards and create an engaging and dynamic
classroom experience for all students. It’s designed in partnership
with Florida teachers to prepare Florida students to be successful
within and beyond the classroom.
FLORIDA
TEACHER GUIDE
CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG/102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG
FLORIDA
TEACHER GUIDE
CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG/102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG

4
Phenomena-Driven Chemistry
for Florida Classrooms
Florida Experience Chemistry brings Chemistry to life with inquiry-based
learning experiences, engaging lab experiences, and customizable options built
specifically for Florida teachers. Our program includes Flinn Scientific
®
labs,
engineering design challenges, and ready-to-go lab kits that save you prep
time and give you the best classroom experience.
Targeted Teaching
Strategies
Use high-impact instructional
strategies to teach with
confidence and make
differentiating instruction easier.
Built on Florida
Standards
Florida Chemistry standards are
clearly defined and at your fingertips.
Less Prep
Time
Simplify lesson planning,
labs, and assessments
with ready-made
Investigation Planners.
Meet Diverse
Needs
Increase science literacy
and accessibility for all
students with targeted
resources and support.
INVESTIGATION 6 PLANNER
Chemical Reactions
In this investigation, students deepen their understanding of energy and matter through a study of
chemical reactions. Students investigate and write reaction equations of combination, decomposition,
single-replacement, double-replacement, and combustion reactions and reactions in aqueous solutions,
as well as other related phenomena.
INVESTIGATIVE PHENOMENON
EXPERIENCE 1 EXPERIENCE 2
Modeling Chemical Reactions
2 periods
Students balance word and chemical equations.
They investigate the law of conservation of energy.
Students analyze data on what causes reactions.
Predicting Outcomes of Chemical Reactions
2 periods
Students interpret data on different types of reactions.
They develop models of the different reactions. They
use models to predict the products of reactions.
CONNECTION TO
THE INVESTIGATIVE
PHENOMENON
Students develop a model for the generation of
energy from the reaction of hydrogen and oxygen
and relate that to the phenomenon of how energy is
obtained from chemical reactions.
Students construct an explanation for the outcome
of a potassium hydroxide and carbon dioxide
reaction and relate that to the phenomenon of how
energy is obtained from chemical reactions.
FLORIDA COURSE
STANDARDS
SC.912.N.1.1, SC.912.P.8.2, SC.912.P.10.1, SC.912.P.10.6,
SC.912.P.10.7, SC.912.P.10.12, SC.912.P.12.12
SC.912.P.8.2, SC.912.P.8.8, SC.912.P.10.12
ENGAGE
Teacher’s Guide Everyday Phenomenon Model a
Chemical Reaction, p. 186
Teacher’s Guide Everyday Phenomenon Elephant
Toothpaste, p. 192
EXPLORE
Inquiry Lab Evaluate Chemical Reactions
Analyzing Data Analyzing Chemical Reactions
Inquiry Lab Types of Chemical Reactions
Virtual Lab Reactivity of Metals
EXPLAIN
Modeling Modeling Chemical Reactions
Animation Bonds Breaking and Forming
Experience Handbook, pp. 226–235
Claim-Evidence-Reasoning Reaction Reasoning
Experience Handbook, pp. 237–249
ELABORATE
Peer Review Rubric Evaluate Models of
Chemical Reactions
Analyzing Data Balance Combustion Equations
Discussion Rubric Classify Reactions and
Predict Their Products
Writing About Science Track the Mass of
Reactants and Products
EVALUATE
Quiz Modeling Chemical Reactions
Experience Handbook Revisit Investigative
Phenomenon, p. 236
Quiz Predicting Outcomes of Chemical
Reactions
Experience Handbook Revisit Investigative
Phenomenon, p. 250
How is energy obtained from
chemical reactions?
Investigative Phenomenon Video Rocket Reaction
Modeling Model the Investigative Phenomenon
Authentic Reading Antarctica’s Blood Red Waterfall
Virtual Reality Experience
Experience Handbook, p. 224
INVESTIGATION ASSETS
182 Investigation 6 Chemical Reactions
CHEM25_TG_FL_INV06_PLN.indd Page 182 13/03/23 9:33 AM user CHEM25_TG_FL_INV06_PLN.indd Page 182 13/03/23 9:33 AM user /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/TG/INV_0 .../102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/TG/INV_0 ...

5
Award-Winning Digital
Platform
Your digital course and classroom is available on Savvas Realize
®
.
With one login, you can access all Florida Standards content,
review planners, assign lessons, and track student progress.
On-Demand Support
Access high-quality program training,
user guides, tips, and more at
MySavvasTraining.com.
Easy Integrations
Customizable and Flexible
Customize lessons, assignments,
playlists, and assessments.
Easy Data Management
Easily retrieve individual and class data to
inform instruction.
• View assignment progress
• Provide feedback
• Transfer scores within the school or district
Schoology
®
Google for Education
Paner

Targeted Strategies Support
Every Student
6
Florida Experience Chemistry gives you the tools to simplify complex topics.
Targeted teaching strategies and resources allow you to reach all learners
and help ensure that every student can be successful in your class.
Differentiated Instruction
You will find ample strategies and tips for
students with special needs, struggling
students, less-proficient readers, and
advanced students.
ELD
Support
Quick tips address
the three English
language development
stages to aid students
learning English.
Remediation
Suggestions
Support students struggling with
specific Chemistry concepts.
Classroom
Modifications
Effective implementation ideas
make activities more open ended or
guided based on students’ abilities.
Address
Misconceptions
Quick tips and
explanations redirect
and promote meaningful
learning.
6
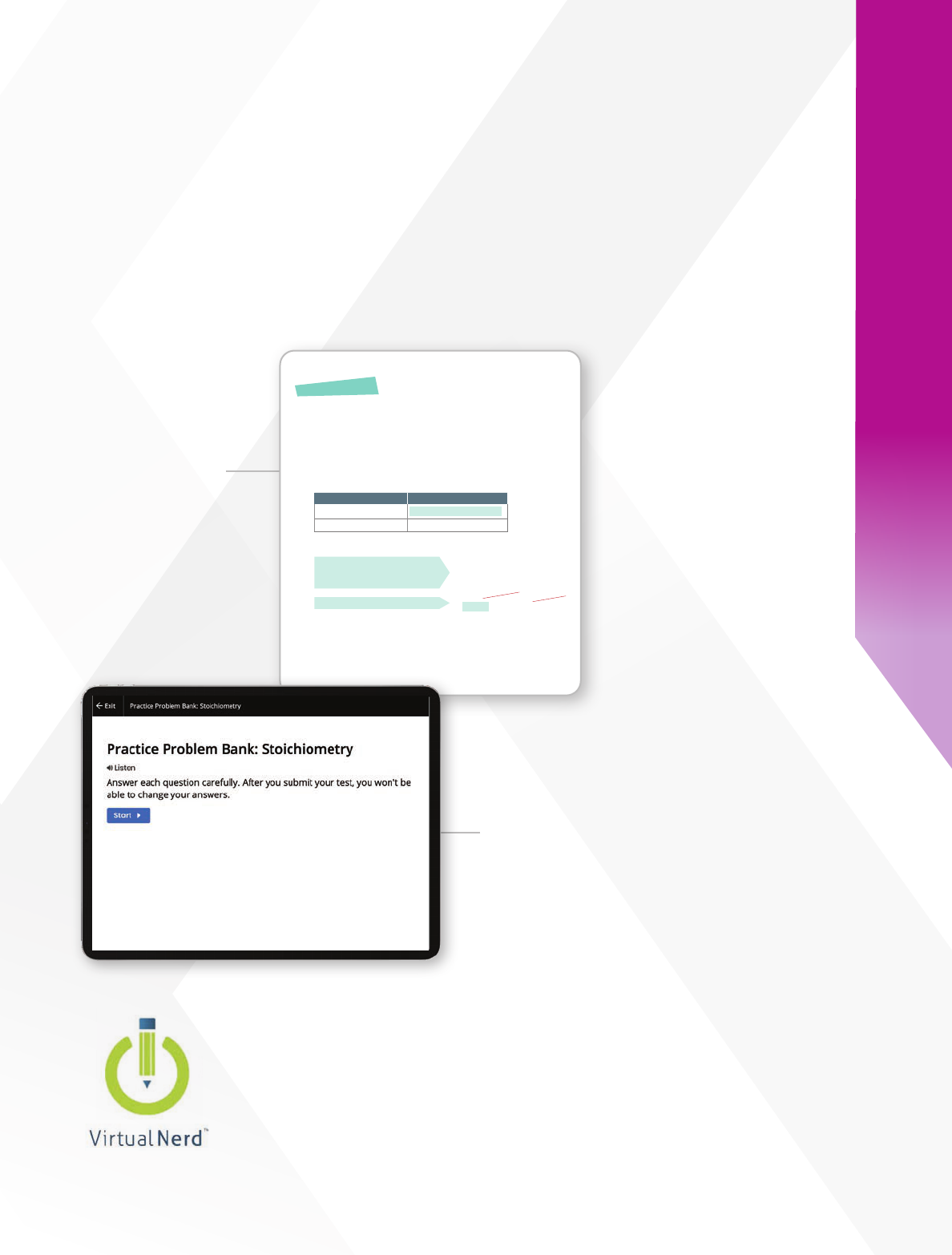
Reinforce Inquiry with Math
Skills Practice
Florida Experience Chemistry provides depth and comprehensive
support to deepen student understanding. The curriculum includes
scaffolding to support scientific reasoning and mathematics to
improve problem solving.
7
Multiple Practice
Opportunties on
Savvas Realize
®
Additional problems build
mathematical fluency.
Virtual Nerd
®
Tutorial Videos
Approachable mathematics
explanations are delivered by
on-screen instructors.
Stepped-Out
Problems
Break down sample
problems for clarity
and guidance.
SUPPORT
SAMPLE PROBLEM
Using Enthalpy of Reaction to
Calculate Enthalpy Change
Calculate the amount of heat (in kJ) required to decompose 2.24 mol of
NaHCO
3
(s).
2NaHCO
3
(s) + 136 kJ → Na
2
CO
3
(s) + H
2
O(g) + CO
2
(g)
ANALYZE List the knowns and the unknown.
Knowns Unknown
amount of NaHCO
3
= 2.24 mol ΔH = ? kJ for 2.24 mol NaHCO
3
ΔH = 136 kJ for 2 mol NaHCO
3
CALCULATE Solve for the unknown.
Use the thermochemical equation to
determine the conversion factor relating
kJ of heat and moles of NaHCO
3
.
Using dimensional analysis, solve for ΔH.
EVALUATE Does the result make sense?
The 136 kJ in the thermochemical equation refers to the decomposition
of 2 mol NaHCO
3
(s). Therefore, the decomposition of 2.24 mol should
absorb more heat than 136 kJ. The answer of 152 kJ is consistent with
theestimate. In addition, the heat is shown on the reactant side of the
equation, which indicates an endothermic reaction. The answer should
be positive for an endothermic reaction (heat absorption), and it is.
10 The production of iron and carbon dioxide from iron(III) oxide and
carbon monoxide is an exothermic reaction. How many kJ of heat
are produced when 3.40 mol Fe
2
O
3
reacts with an excess of CO?
Fe
2
O
3
(s) + 3CO(g) → 2Fe(s) + 3CO
2
(g) + 26.3 kJ
= 152 kJ
GO ONLINE for more practice problems.
136 kJ
_____________
2 mol NaHCO
3
ΔH = 2.24
mol NaHCO
3
×
136 kJ
______________
2 mol NaHCO
3
ΔH = 3.40 mol Fe
2
O
3
(s) ×
−26.3 kJ
_____________
1 mol Fe
2
O
3
(s)
= −89.4 kJ
1 Energy in Chemical Bonds 299
CHEM25_SE_FL_INV08.indd Page 299 22/02/23 9:46 AM f-0401-mac CHEM25_SE_FL_INV08.indd Page 299 22/02/23 9:46 AM f-0401-mac /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/SE/INV_0 .../102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/SE/INV_0 ...
Lab Analyses
• Deeper understanding of
Chemistry concepts
• Scientific and engineering skills
into practice
• Simulation of real lab experiences
Analyze Data
Activities
Promote scientific thinking
as each student:
• Applies mathematical
concepts in real-world
contexts
• Enhances critical
thinking skills
• Promotes scientific
inquiry

8
Integrated Nature of
Science Standards
You can teach with confidence knowing that the Florida Nature of Science
standards are integrated throughout the course in Inquiry Labs, Analyzing Data
activities, Engineering Design Challenges, and other activities.
NAME
DATE
CLASS
NATURE OF SCIENCE
Data: Analysis and Calculations
Synthesize Information
1. SEP Organize Data For the data sets in the following investigations, choose the
best format to use to organize the quantitative and qualitative data: bar chart, line
graph, scatter plot, data table, or labeled diagrams. (There may be more than
one correct answer.)
a. Examining the skeletal structures of different species of frogs
b. Using multiple trials to measure the optimal temperature at which a
chemical reaction occurs
c. Cataloging the number of people in different age bands (0–4, 5–9, 10–14,
etc.)
d. Looking for patterns in the relationship between the sizes of adult birds of
ten different species and the sizes of their eggs
e. Showing the distance a car has traveled as a function of time
2. SEP Analyze Data For the investigation of the cooling rate of a cup of coffee in
the Nature of Science reading:
a. Identify and describe the pattern observed for the rate of cooling.
b. Describe which method of organizing the quantitative data made it easiest
to identify this pattern and explain why.
3. SEP Analyze Data In 2022, the U.S. Environmental Protection Agency (EPA)
tested the ranges of driving for many electric cars available on the market. Test
the hypothesis that the range of driving for electric cars is positively correlated
with the size of the battery (in kilowatt-hours, or kWh). For the following six
electric car models tested by the EPA, plot the provided data on a graph. Plot the
range (in km) along the y-axis and the battery capacity (in kWh) along the x-axis.
Copyright © Savvas Learning Company LLC. All Rights Reserved.
Savvas is not responsible for any modifications made by end users to the content posted in its original format.
NAME
DATE
CLASS
Car Range (km) Battery Capacity (kWh)
1 837 118
2 161 36
3 563 108
4 415 64
5 499 77
6 346 62
a. Draw a line of best fit for your set of six points. Then identify and describe
the statistical correlation between battery capacity and range of driving for
electric cars.
b. Which of the six data points would you consider an outlier? Explain.
c. Explain how removing the outlier in part (b) would change the calculation
of the R value. (Recall that an R value, which ranges from −1 to 1, is a
quantitative statistical measure for how strong the linear relationship is
between two variables.)
d. Propose an explanation for why all the points do not fall upon a straight
line.
4. SEP Use Mathematical Calculations For each of the following equations,
assess the quantitative relationship between the two indicated variables and tell
whether the relationship is linear, inverse, quadratic, or exponential. (In each
case, the quantity k is a constant that does not change.)
a. The relationship between pressure P and volume V in
b. The relationship between volume V and temperature T in V = kT
c. The relationship between the number of bacteria N and time t in
d. The relationship between kinetic energy KE and speed S in
e. The relationship between energy E and mass m in E = mk
Copyright © Savvas Learning Company LLC. All Rights Reserved.
Savvas is not responsible for any modifications made by end users to the content posted in its original format.
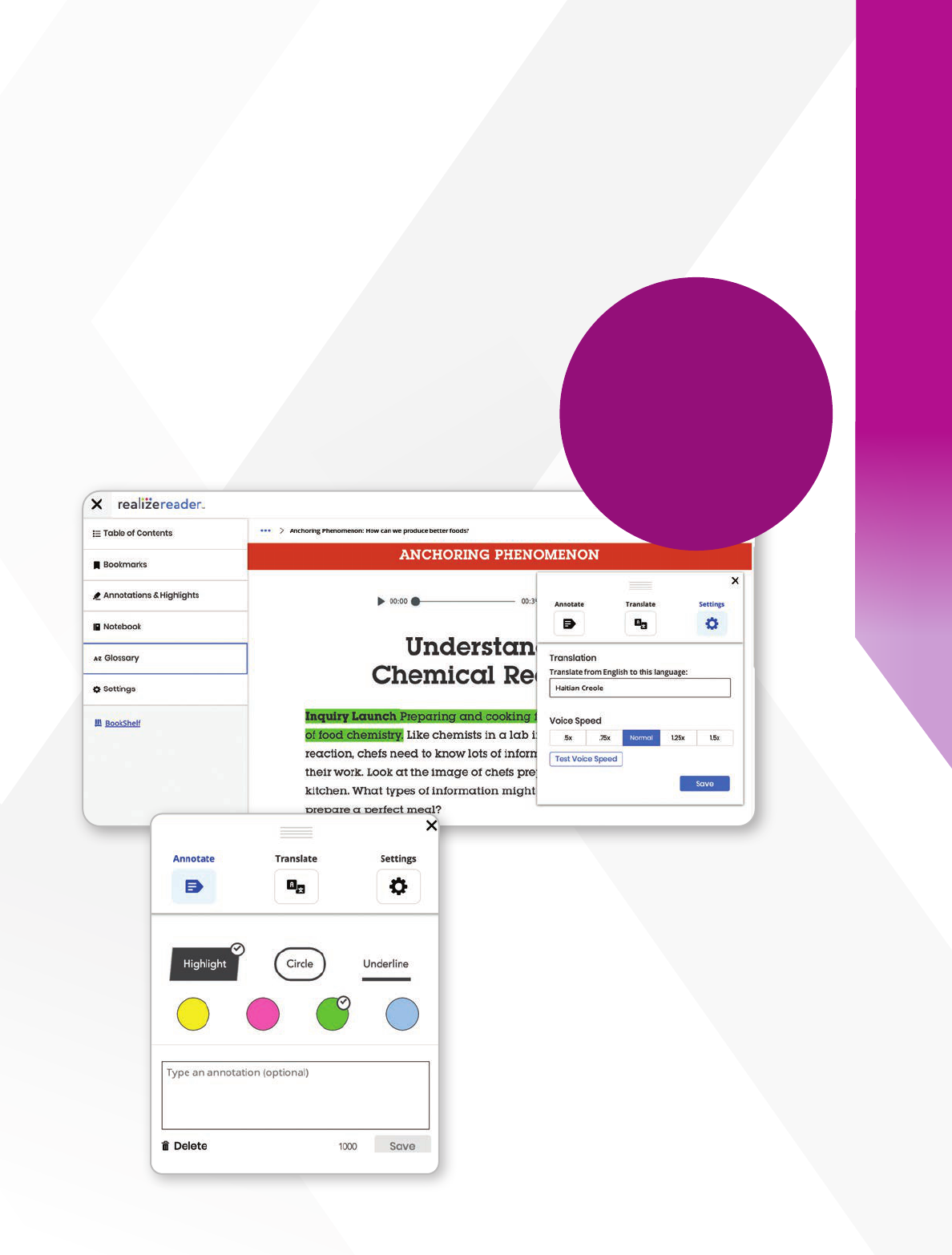
Boost Accessibility
Integrated assistive technology on Savvas
Realize
®
allows students to interact with the
text and personalize their learning, improving
accessibility for all learners. Students also have
access to the course glossary to enhance
their understanding of key terms and
concepts while reading.
9
Embedded
Annotation Tool
Help students more actively
engage with the text and
improve their comprehension
and critical reasoning skills.
SUPPORT
Translate
e-text
into 100+
languages
with TextHelp

10
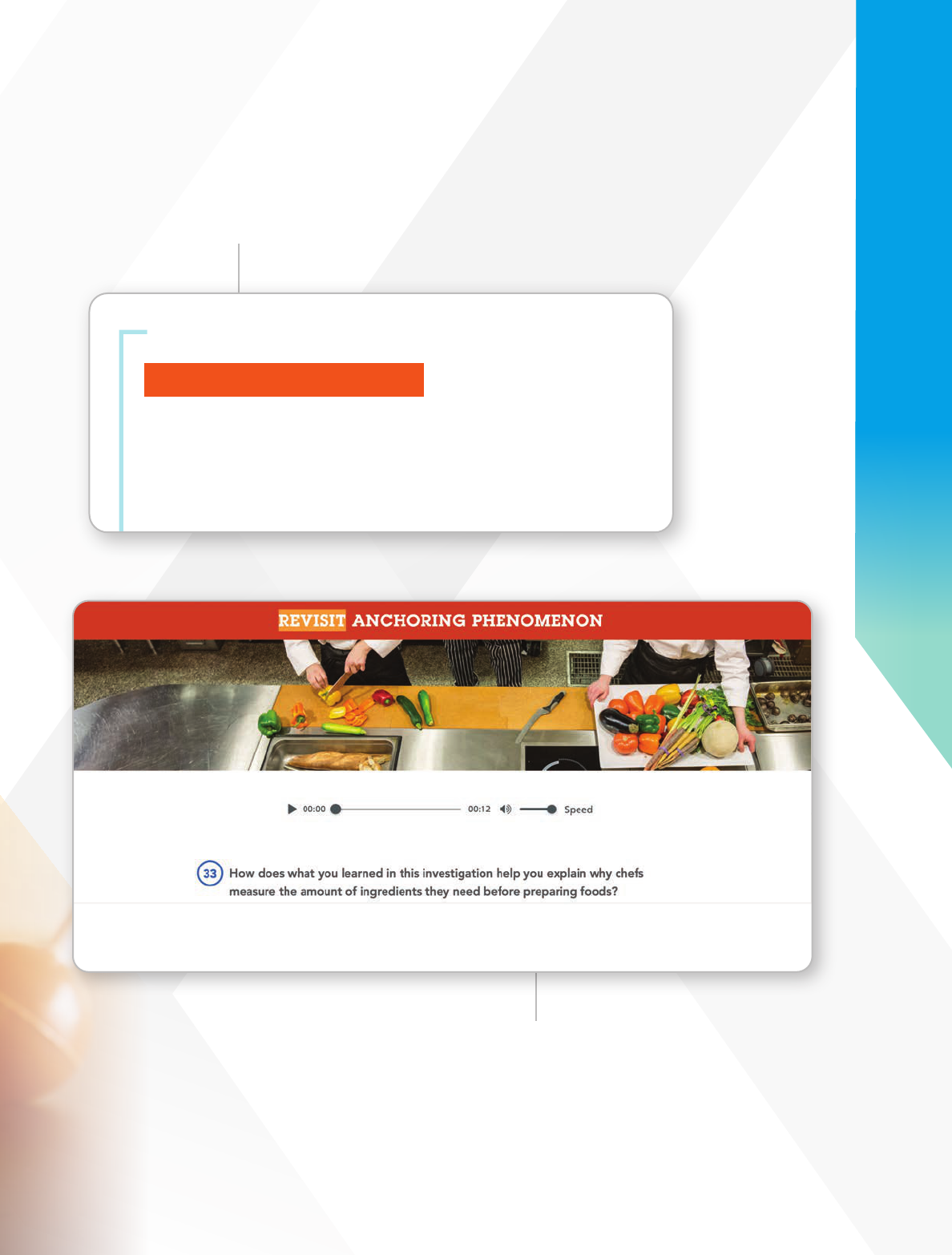
Phenomena-Based Science
Connects to Everyday Experiences
Florida Experience Chemistry connects
Chemistry concepts to real life with
Anchoring Phenomena, Investigations,
and Experiences. This approach makes
learning more relevant and easier to
understand for students.
Phenomena Launch
Start with a Phenomenon Video,
Authentic Reading, or Demonstration
to help connect Chemistry concepts
with real world applications.
Student Sensemaking
Help improve students’ reasoning
and critical thinking skills through
hands-on experiences using the
Modeling Worksheet or Claim-
Evidence-Reasoning (CER) model.
11
Phenomena-Based Science
Connects to Everyday Experiences
ENGAGE
EVERYDAY PHENOMENON
RELATED PHENOMENA
In addition to the demo, Model a Chemical
Reaction, consider using other phenomena to
launch this experience.
• Hot Versus Cold Provide a hot pack and
a cold pack. Have students feel the two.
Discuss the chemical equations for both:
cold pack—NH
4
NO
3
(s) + H
2
O(l ) → NH
4
+
(aq)
+ NO
3
−
(aq); hot pack—CaCl
2
(s) + H
2
O(l ) →
Ca
2+
(aq) + 2Cl
−
(aq). Discuss which shows an
endothermic reaction and which shows an
exothermic reaction.
• Crime Scene Glow Slowly mix 500 mL of
luminol solution with 500 mL of household
bleach solution. Dim the lights and watch
as it generates a blue glow. Discuss what
caused the reaction and whether it was
exothermic or endothermic.
Does the number of atoms change in a
chemical reaction?
Use this teacher demo to prompt student discussion about chemical reactions
and equations. As students ask questions about this phenomenon, challenge
them to find answers as they complete the Inquiry Lab.
Model a Chemical Reaction
Engage students with this demonstration of an everyday phenomenon to
introduce the concept of chemical reactions and how models can be used to
illustrate energy in a chemical reaction system.
Materials 100-mL graduated cylinder, 0.1 M copper(II) chloride solution,
400-mL beaker, metric ruler, scissors, 5-cm square piece aluminum foil
Safety Copper(II) chloride solution is an irritant.
1. Add 200 mL of 0.1 M copper(II) chloride solution to a 400-mL beaker.
2. Crumple the foil into a loose ball and place it in the copper solution. Ask
students to note any evidence of chemical change. (Students should note
that the solution begins to produce gas and that a red-brown precipitate of
copper falls to the bottom of the beaker.)
3. Have students write a sentence in their notebooks that describes the
chemical reaction. Then explain that students can also model what happens
in a chemical reaction using word equations and drawings. Write the skeleton
equation for the reaction on the board: CuCl
2
+ Al → AlCl
3
+ Cu.
Modeling Chemical Reactions
EXPERIENCE 1
Teacher Background
Experience 1 guides students as they model chemical reactions, starting with
word equations. Students write skeleton equations and decode symbols that
are used in chemical equations. They use mathematical and computational
thinking to practice balancing chemical equations. They analyze relationships
between reactants and products and interpret data on what causes reactions,
using knowledge of the collision theory. Students may have difficulty accepting
the collision theory because they tend to visualize atoms and molecules wedged
together with no spaces between them. Remind students of their previous
knowledge of the kinetic theory—that all matter is made of tiny particles that
are in constant motion, and that between the particles is empty space.
Objectives
• Write balanced equations for chemical reactions.
• Distinguish between endothermic reactions and exothermic reactions.
• Explain what causes chemical reactions.
• Develop a basic conceptual and mathematical model for the generation
of energy from the reaction of two substances.
FLORIDA CHEMISTRY 1
COURSE STANDARDS
SC.912.N.1.1 Define a problem based
on a specific body of knowledge, for
example: biology, chemistry, physics, and
earth/space science, and do the following: 9.
Use appropriate evidence and reasoning to
justify these explanations to others.
SC.912.P.8.2 Differentiate between
physical and chemical properties and
physical and chemical changes of matter.
SC.912.P.10.1 Differentiate among the
various forms of energy and recognize that
they can be transformed from one form to
others
SC.912.P.10.6 Create and interpret
potential energy diagrams, for example:
chemical reactions, orbits around a central
body, motion of a pendulum.
SC.912.P.10.7 Distinguish between
endothermic and exothermic chemical
processes.
SC.912.P.10.12 Differentiate between
chemical and nuclear reactions
SC.912.P.12.12 Explain how various
factors, such as concentration,
temperature, and presence of a catalyst
affect the rate of a chemical reaction.
Also: MA.K12.MTR.2.1
186 Investigation 6 Chemical Reactions
CHEM25_TG_FL_INV06.indd Page 186 17/03/23 11:42 AM user CHEM25_TG_FL_INV06.indd Page 186 17/03/23 11:42 AM user /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/TG/INV_0 .../102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/TG/INV_0 ...
Everyday
Phenomenon
Students learn the reasons
behind naturally occurring
phenomena.
Revisit Anchoring Phenomenon
Promote understanding with multiple opportunities
to revisit core concepts and skills.
ENGAGE

12
Flinn Scientific Takes Inquiry
to a Higher Level
An exclusive partnership with Flinn Scientific
®
, the leading provider of science
lab solutions, delivers exceptional quality to your classroom. Flinn Scientific Labs
are embedded directly into Florida Experience Chemistry.
Inquiry Labs
Every learning experience includes a
hands-on inquiry lab developed by Flinn
Scientific. Each lab has four options, so
you can easily differentiate instruction.
NAME
DATE
CLASS
Copyright © 2019 Flinn Scientific, Inc. All Rights Reserved.
Flinn Scientific and its affiliates are not responsible for any modifications made by end users to the content posted in its original format.
INQUIRY LAB – SHORT
Metal Activity
How are the activity series of metals explored? The purpose of this experiment is to
carry out a series of possible single replacement reactions of metals with solutions of
metal cations in order to determine the activity series of the metals. Which metal is most
active?
Focus on Science Practices
SEP 3 Planning and Carrying Out Investigations
SEP 5 Using Mathematics and Computational Thinking
Materials Per Group
● Copper, Cu, 1-cm
2
strips, 2
● Forceps or tweezers
● Copper(II) sulfate solution, CuSO
4
,
0.2M, 4 mL
● Iron, Fe, 1-cm
2
strips, 2
● Marking pen
● Paper towels
● Pipets, Beral-type, 3
● Test tube, small, 4
● Silver nitrate solution, AgNO
3
,
0.2M, 4 mL
● Ruler
● Sandpaper (optional)
● Zinc sulfate solution, ZnSO
4
,
0.2M, 4 mL
● Distilled water and wash bottle
● Test tube rack
Safety
Silver nitrate is slightly toxic by ingestion and will stain skin and clothing. Copper(II)
sulfate is toxic by ingestion. Metal pieces may have sharp edges—handle with care.
Avoid contact of all chemicals with eyes and skin. Wear chemical splash goggles,
chemical-resistant gloves, and a chemical-resistant apron. Wash hands thoroughly with
soap and water before leaving the lab.
Procedure
1. Obtain four small test tubes and place on the test tube rack. With the marking
pen, label each 1 to 4.
Ready-Made Lab Kits
Simplify setup with time-saving, readily
accessible kits from Flinn Scientific.
4 Options for
ALL Inquiry Labs
• Open-Ended
• Guided
• Shortened
• Advanced

13
Flinn Scientific Takes Inquiry
to a Higher Level
Virtual Reality
Experiences
360º online lab simulations
extend inquiry. Students can
experiment with premium lab
equipment and safely deal
with chemicals.
Engineering
Design Challenges
Students will design, test,
and evaluate solutions to
improve their science and
engineering skills.
NAME
DATE
CLASS
ENGINEERING DESIGN CHALLENGE
Design a Green Roof
When you look at the building materials used in cities, you see lots of dark surfaces
like asphalt and brick. Materials like these have much lower albedos, especially when
comparing them to the natural surfaces that existed prior to the construction of the city.
Since they have a lower albedo, they absorb more of the energy emitted from the Sun,
causing them to warm up. As a result, during the summer, cities can be as much as
12°C warmer than the rural areas located next to them. This phenomenon is called the
urban heat island effect. The urban heat island effect contributes to increases in air
pollution, energy consumption, and heat-related illness to mention a few. What if you
could redesign some of these surfaces to absorb less of the Sun’s energy, lowering air
temperature while improving air quality in major cities? You will design a green roof to
accomplish just that.
Focus on Engineering Practices
SEP 1 Defining Problems
SEP 3 Planning and Carrying Out Investigations
SEP 6 Designing Solutions
Materials Per Group
● Alfalfa seed, 3 g
● Barley seed, 3 g
● Construction paper, black, 21.59
cm by 27.94 cm, 1
● Foam, 20.3 cm by 20.3 cm, 2
● Foam, 20.3 cm by 22.9 cm, 2
● Grass seed, 3 g
● Heat lamp
● Mustard seed, 3 g
● Oat seed, 3 g
● Plastic container
● Soil, 700 g
● Thermometer
● Toothpicks, 8
Copyright © 2019 Flinn Scientific, Inc. All Rights Reserved.
Flinn Scientific and its affiliates are not responsible for any modifications made by end users to the content posted in its original format.
Lab Videos
Bite-sized, captivating video
clips deepen understanding
and enrich lab experiences.
Performance-Based
Assessments
Leverage alternative assessment
options with opportunities for
students to demonstrate mastery
of the Florida Standards.
ENGAGE
360
º

14
Engage Minds with
Active Learning
PhET
™
Simulations
Interactive simulations make
it easier for students to grasp
concepts without expensive
equipment.
Virtual Labs
Access compelling phenomena
and advanced scientific
equipment.
Unlock the POSSIBLE in science with our blend of dynamic hands-on and
virtual activities. These activities encourage students to think critically about
how Chemistry concepts apply to the world around them.

15
Problem-Based
Learning Activity
Students define problems and
propose design solutions.
Animations
Explanations of Chemistry
concepts are provided in
easy-to-grasp demonstrations.
ENGAGE
Modeling Activity
Students develop models, just like
actual scientists, as they explore
chemical phenomena.
NAME
DATE
CLASS
MODELING
Model Electron Configurations
Electron configurations can be represented in a variety of ways. Scientists often use
boxes containing arrows to represent electrons in atomic orbitals, as shown in the table.
The same information can be represented by a notation consisting of numbers, letters,
and superscripts.
Element 1s 2s 2p
x
2p
y
2p
z
Electron configuration
N ↑↓ ↑↓ ↑ ↑ ↑ 1s
2
2s
2
2p
3
O ↑↓ ↑↓ ↑↓ ↑ ↑ 1s
2
2s
2
2p
4
Develop and Use Your Model
1. SEP Develop a Model Use the space to develop your ideas for a new model,
that is different from the table that is shown, for the electron configuration of
either nitrogen (N) or oxygen (O). Be sure to consider the following criteria:
● Show each electron and identify its atomic orbital.
● Clearly distinguish the outermost, or valence, electrons.
● Include labels and/or captions to make your meaning clear.
Copyright © Savvas Learning Company LLC. All Rights Reserved.
Savvas is not responsible for any modifications made by end users to the content posted in its original format.
Visual Analogies
Located in the Student Handbook,
infographics help students
understand complex Chemistry
concepts.

Enrichment for Mastery
Got More Time?
Assign extension activities to maximize instruction
and real-world application of Chemistry concepts.
GOT MORE TIME? Personalize and enhance your instructional plan by assigning
the activities with the got-more-time icon, as time allows.
Performance-Based Assessment Microhabitat in a Bottle
3-D Assessment Weather and Climate
Experience Handbook Performance-Based Assessment, p. 413;
Revisit Anchoring Phenomenon, p. 413; Appendix C Problem
Bank, p. R23
INSTRUCTIONAL SEGMENT ASSESSMENT
EXPERIENCE 3 EXPERIENCE 4 EXPERIENCE 5
Atmospheric System Feedbacks
2 periods
Students construct an explanation for why
croplands would have a higher albedo than
forests. Students design a solution for how
humans can reduce carbon dioxide.
Long-Term Climate Factors
2 periods
Students construct an explanation about
what will happen to the ocean as the global
temperature increases.
Short-Term Climate Factors
2 periods
Students use computational thinking to
calculate and explain Earth’s exposure to the
Sun’s rays.
Students analyze evaporation feedbacks
and use this to refine explanations on what
causes drought in California.
Students use their knowledge of climate
zones to refine explanations on what causes
drought in California.
Students apply knowledge of how humans
have impacted climate to refine explanations
on what causes drought in California.
SC.912.P.10.18, SC.912.N.1.1, MA.K12.MTR.6.1 SC.912.N.1.1, MA.K12.MTR.7.1 SC.912.N.1.1, MA.K12.MTR.7.1
Teacher’s Guide Everyday Phenomenon
Counting Pennies, p. 305
Teacher’s Guide Everyday Phenomenon
Frozen Balloons, p. 311
Teacher’s Guide Everyday Phenomenon
Spin Cycle, p. 317
Inquiry Lab Albedo and Composition of
Earth’s Surface
Interactivity Wetlands and the
CarbonCycle
Inquiry Lab How Melting Ice Affects
Sea Level
Analyzing Data Historical Carbon
Dioxide Levels
Inquiry Lab Observe Air Pollution
Virtual Lab Sampling the Past
Claim-Evidence-Reasoning Discuss the
Wetland Effect
Animation Cold and White: A
Reinforcing Feedback Loop
Experience Handbook, pp. 382–391
Claim-Evidence-Reasoning Heat
Expansion
Experience Handbook, pp. 392–400
Modeling Milankovitch Cycles
Experience Handbook, pp. 401–411
Discussion Rubric Beavers Decrease
Temperature Swings
Engineering Design Challenge
Design a Green Roof
Peer Review Rubric Evaluate Predicted
CO
2
Levels
Writing About Science Snowball Earth
Peer Review Rubric Evaluate
Milankovitch Cycles
Analyzing Data Solar Output
Quiz Atmospheric System Feedbacks
Experience Handbook Revisit Investigative
Phenomenon, p. 391
Quiz Long-Term Climate Factors
Experience Handbook Revisit Investigative
Phenomenon, p. 400
Quiz Short-Term Climate Factors
Experience Handbook Revisit
Investigative Phenomenon, p. 412
289
CHEM25_TG_FL_INV10_PLN.indd Page 289 20/03/23 12:40 PM f-0315 CHEM25_TG_FL_INV10_PLN.indd Page 289 20/03/23 12:40 PM f-0315 /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/TG/INV_1 .../102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/TG/INV_1 ...
Florida Experience Chemistry offers multiple enrichment opportunities
for all students to master core Chemistry concepts through impactful
extension activities.
16

17
Enrichment
Opportunities
Deepen core concept knowledge and
extend the learning with:
• Write About Science
• Discussion and Peer Review Rubrics
• Problem-Based Learning Activities
• Authentic Reading
• Analyzing Data Activities
• Biographies
PREPARE
NAME
DAT E
CLASS
WRITING ABOUT SCIENCE
Compare Acid-Base Models
Scientists’ descriptions of acids and bases changed over time. In 1887, Svante
Arrhenius was one of the first people to define acids and bases on a molecular level.
The Brønsted-Lowry model was proposed in 1923. Gilbert Lewis separately developed
his model in 1923. Use information gathered from authoritative sources on your own to
answer Questions 1 and 2.
Obtain and Evaluate Information
1. Connect to Nature of Science Conduct research to learn about the three models
of acids and bases. Describe each scientist’s definition and the differences
between them.
2. Connect to Nature of Science Based on your understanding of the three
definitions, do they contradict each other or do they build on one another? Use
evidence to explain your answer.
Copyright © Savvas Learning Company LLC. All Rights Reserved.
Savvas is not responsible for any modifications made by end users to the content posted in its original format.
NAME
DAT E
CLASS
PROBLEM-BASED LEARNING
Minerals, Crystals, and Gemstones
Fireworks are different colors because of the elements they contain. The same is true of
gemstones. The only difference between a piece of clear corundum crystal and a
beautiful red ruby is a slight inclusion of the element chromium. If the corundum crystal
had titanium instead, it would be a beautiful blue sapphire!
Over the course of this activity, you will develop your knowledge of crystal structures
and colors. First, you will explore the colors of elements when they are in ions in water
solutions. Then, you will explore the colors of elements of the periodic table when they
are included in crystals and gems. Finally, you will explore crystal structures and then
grow a crystal of your own.
As this research project will include many beautiful, colored photos and crystals,
consider whether you would like to present your research throughout this project as a
table, Web page, computer-based presentation, or poster.
Define the Problem: How do the elements present in crystals and
gems affect their structure and properties?
Part 1 –– Color and Charge
Not only do different elements have different colors, but some elements can form more
than one color depending on their charge. In other words, even just changing the
charge on an ion can lead to a dramatic change in the color of compounds it forms.
1. SEP Collect Data Research the transition metals chromium (Cr), manganese
(Mn), and vanadium (V) and record the colors that each element can create in
aqueous solution depending on their charge. Create a table, Web page,
computer-based presentation, or poster to summarize this data and include
colored images of solutions containing these elements.
Copyright © Savvas Learning Company LLC. All Rights Reserved.
Savvas is not responsible for any modifications made by end users to the content posted in its original format.
NAME
DATE
CLASS
Copyright © by Savvas Learning Company LLC. All Rights Reserved.
1
INVESTIGATIVE PHENOMENON: AUTHENTIC READING
Boring Sponges
In acidic water, drilling sponges damage scallops twice as quickly,
worsening the effects of ocean acidification
by Hannah Waters
Whenever
anyone talks about ocean acidification, they discuss vanishing corals and other
shelled organisms. But these aren’t the only organisms affected—the organisms that interact
with these vulnerable species will also change along with them.
These changes won’t necessarily be for the good of the shell and skeleton builders. New
research published in Marine Biology shows that boring sponges eroded scallop shells twice as
fast under the more acidic conditions projected for the year 2100. This makes bad news for the
scallops even worse: not only will they have to cope with weakened shells from acidification
alone, but their shells will crumble even more quickly after their cohabiters move in.
Cliona celata (yellow), a boring sponge species, lives throughout the Atlantic and Mediterranean. Here, numerous
sponges have drilled into coral.
Boring sponges aren’t named thus because they’re mundane; rather, they make their homes by
boring holes into the calcium carbonate shells and skeletons of animals like scallops, oysters
and corals. Using chemicals, they etch into the shell and then mechanically wash away the tiny
shell chips, slowly spreading holes within the skeleton or shell and sometimes across its
surface. Eventually, these holes and tunnels can kill their host, but the sponge will continue to
live there until the entire shell has eroded away.
Copyright © by Savvas Learning Company LLC. All Rights Reserved.
Savvas Learning Company is not responsible for any modifications made by end users to the content posted i n its original format.
Sangeeta Bhatia, M.D., Ph.D.
Bioengineer
Sangeeta Bhatia is an Indian-American cancer researcher, MIT professor, and biotech
entrepreneur. Her parents immigrated from India to Boston, Massachusetts when she was
a young girl. She was inspired to pursue a career in engineering after her tenth-grade
biology class and visiting an MIT research lab with her father, who was also an engineer.
Dr. Bhatia is unique because she is trained as both a physician and engineer, with
degrees from Harvard, MIT, and Brown University.
Her laboratory at MIT leverages ‘tiny technologies’ of miniaturization to yield inventions
with new applications in tissue regeneration, stem cell differentiation, medical diagnostics,
predictive toxicology, and drug delivery. Some of their inventions include human micro-
livers that can model drug metabolism, liver disease, and the liver’s interaction with
pathogens. They have also created nanosensors that can detect tumor tissue types,
which leads to earlier detection of cancerous tumors. Bhatia hopes that this will one day
revolutionize how cancer is detected and treated. In 2015, she gave a TED talk about this
technology.
She and her trainees have created more than 40 issued or pending patents through their
research. They have also launched multiple biotechnology companies to improve human
health through the use of miniaturization in medicine. Bhatia is an active mentor and
advocate for diversity in her fields. She was the recipient of the Harvard Medical School
Diversity Award and the Harvard-MIT Thomas McMahon Mentoring award. Other honors
include the Lemelson-MIT Prize, known as the ‘Oscar for inventors,’ and the Heinz Medal
for groundbreaking inventions and advocacy for women in STEM fields.

18
Prepare for Success
Raise the bar! Multiple and varied modes
of assessment allow students to better
demonstrate their understanding of the
Florida Science Standards.
Program Level Assessments
• Problem-Based Learning Experiences
• ExamView
®
Assessment Bank
• Pre/Post Test
Investigation Level
Assessments
• Performance-Based Assessments
• Investigation Assessments
• Problem Bank
Assign
and edit
assessments
on Savvas
Realize
®
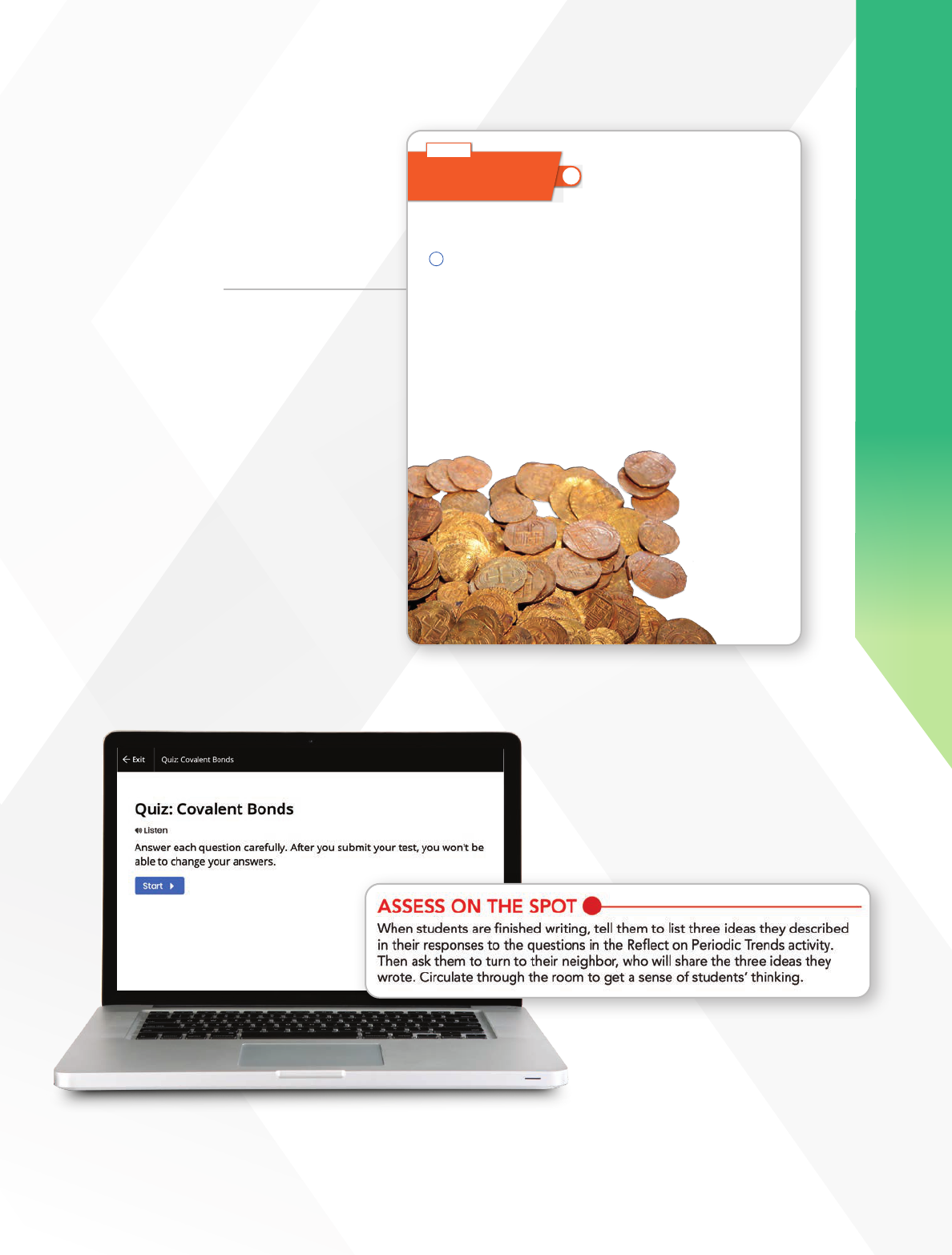
19
PREPARE
Experience Level
Assessments
• Interactive Online Quizzes
• Assess-on-the-Spot
• Revisit Investigative Phenomena
• Formative Observations
INVESTIGATIVE
PHENOMENON
Revisit
In the CER worksheet, you constructed an argument to explain how electron
shielding changes across a period or within a group. With a partner,
reevaluate the evidence cited in your arguments.
19 Refine Your Argument How could you expand your argument to
support periodic trends? Explain.
GO ONLINE to Elaborate on and Evaluate your
knowledge of periodic trends by completing the
engineering design and class discussion activities.
Sample answer: Electron shielding affects the effective nuclear charge of an
atom. The trends observed for electron shielding and effective nuclear charge can
be used to understand the trends of other elemental properties. For example,
since effective nuclear charge increases from left to right across a period the
energy needed to remove an electron (ionization energy) also increases. Similarly,
effective nuclear charge stays about the same within a group. But valence
electrons are farther aways from the nucleus moving down a group so ionization
energy decreases down a group.
74 Investigation 2 The Periodic Table
CHEM25_SE_FL_INV02.indd Page 74 22/02/23 9:06 AM f-0401-mac CHEM25_SE_FL_INV02.indd Page 74 22/02/23 9:06 AM f-0401-mac /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/SE/INV_0 .../102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Interior_Files/SE/INV_0 ...

FLORIDA
Savvas.com
800-848-9500
Copyright © 2023 Savvas Learning Company LLC. All Rights Reserved. Savvas
®
and Savvas Learning Company
®
are the registered trademarks of Savvas Learning Company LLC in the US and in other countries.
Unless otherwise indicated herein, all third-party trademarks are the property of their respective owners and are
not intended to imply any sponsorship or endorsement by the owners of such trademarks.
SAM: 9781428557932 ADV: 9781428557963
Experience the
POSSIBLE.
Florida Experience Chemistry is
an exciting phenomena-based
curriculum that helps you deliver
standards-aligned instruction. An
exclusive partnership with Flinn
Scientic
®
offers the highest quality
labs and clear pathways for
differentiation. With this program,
it IS possible to transform your
classroom! Speak to your Savvas
representative to learn more.
Contact your Savvas Representative for support or visit:
Savvas.com/FLScience
0523.LB.P2.Sci581R983 Photos © Shutterstock
Join the Conversation
@SavvasLearning
Get Fresh Ideas for Teaching
Blog.Savvas.com
FLORIDA
TEACHER GUIDE
CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG/102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG
FLORIDA
TEACHER GUIDE
CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac CHEM25_TG_FL_ENG_FCVR.indd Page 1 28/03/23 3:21 PM f-0401-mac /102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG/102/SA00969/EXPCHEM25/FL/SE/2022/G9-12/1428527850/Layout/Cover/TG
