
Petri Dish Electrolysis
Electrolysis Reactions
Introduction
Electrolysis is defined as the decomposition of a substance by means of an electric current. When an electric current is
passed through an aqueous solution containing an electrolyte, the water molecules decompose via an oxidation–reduction
reaction. Oxygen gas is generated at the anode, hydrogen gas at the cathode. Depending on the nature of the electrolyte,
different reactions may take place at the anode and the cathode during the electrolysis of aqueous solutions. Build simple
and inexpensive electrochemical cells using Petri dishes to compare the reactions of sodium sulfate, potassium iodide, and
tin(II) chloride.
Concepts
• Electrolysis • Oxidation and reduction
• Anode and cathode • Acid–base indicators
Materials
Phenolphthalein indicator solution, 0.5%, 1 mL Beral-type pipets, 2
Potassium iodide solution, KI, 0.5 M, 20 mL Paper clips, 2
Sodium sulfate solution, Na
2
SO
4
, 0.5 M, 20 mL Paper towels
Starch solution, 0.5%, 1 mL Pencil leads, 7 mm, 2
Tin(II) chloride solution, acidified, SnCl
2
, 1 M, 20 mL* Petri dish
Universal indicator solution, 5 mL Stirring rod
9-V Battery Wash bottle and distilled water
Battery cap w/ alligator clip leads
*See the Preparation section.
Safety Precautions
The acidic tin(II) chloride solution is corrosive to body tissue and moderately toxic by ingestion. Phenolphthalein and universal indicators
solutions contain ethyl alcohol and are flammable liquids. Keep away from flames and heat. Avoid contact of all chemicals with eyes and
skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Wash hands thoroughly with soap and
water before leaving the lab. Please review current Material Safety Data Sheets for additional safety, handling, and disposal
information.
Preparation
Prepare acidified 1 M tin(II) chloride solution by dissolving 22.5 g of tin(II) chloride dihydrate (SnCl
2
z2H
2
O) in 100 mL of
1 M hydrochloric acid.
Procedure
1. Place a clean Petri dish on the overhead projector stage and carefully pour about 20 mL of 0.5 M sodium sulfate
solution into the dish.
2. Add 2–3 mL of universal indicator solution and stir to mix. The solution should be a rich, transparent green color
(neutral pH, 7).
3. Break a pencil lead in half. Attach the two pencil leads to opposite sides of the Petri dish with the alligator clips on the
battery cap. Connect the battery cap to the 9-V battery (see Figure 1).
© 2016 Flinn Scientific, Inc. All Rights Reserved. 1
Publication No. 95008
061616
SCIENTIFIC

Petri Dish Electrolysis continued
2
© 2016 Flinn Scientific, Inc. All Rights Reserved.
Demonstration
Solution
Pencil lead
Petri Dish Bottom
9 V
Clip
Battery
Alligator
Clip
Figure 1. Demonstration Setup.
4. Make sure each pencil lead is submerged in the sodium sulfate solution. Try to keep the alligator clips out of the liquid if
possible.
5. Let the demonstration run for 3–5 minutes. Observe and record all changes as the current flows through the electrolysis
solution. Be specific—compare the changes at the pencil leads attached to the positive (+) and negative (–) terminals of the
battery. Note: The conducting surfaces at which electric current passes into and out of the solution are called electrodes.
The electrode at which oxidation occurs is called the anode, while the electrode at which reduction occurs is called the
cathode. In an electrolytic cell, the anode is (+) and the cathode is (–). The general convention is to show the anode on the
left and the cathode on the right in figures of an electrochemical cell. Following this convention when performing this
demonstration will help avoid confusion.
6. Note the changing colors over time. A purple color will appear the cathode and an orange color will appear at the anode.
Soon the entire spectrum of universal indicator color changes may be visible. What pH changes are responsible for the
various colors?
7. Observe the formation and appearance of gas bubbles at each electrode. Can you identify which gas is hydrogen and which
is oxygen based on the rate of bubbling and the apparent solubility of the gases in the solution?
8. Disconnect the alligator clips from the Petri dish and carefully remove the carbon pencil leads from the solution. Rinse the
pencil leads with distilled water from a wash bottle and gently pat dry with a paper towel.
9. Stir the sodium sulfate solution in the Petri dish and observe the final indicator color of the solution after mixing. It should
be green, corresponding to no net production of H
+
or OH
–
ions in the electrolysis of water.
10. Pour the solution from the Petri dish into a waste beaker and rinse well with distilled water.
11. Place the clean Petri dish on the overhead projector stage and carefully add about 20 mL of 0.5 M potassium iodide
solution.
12. Add 3–5 drops of phenolphthalein solution to the potassium iodide solution and stir to mix.
13. Attach clean pencil lead electrodes to each alligator clip lead on the battery cap and submerge the electrodes into the KI
solution.
14. Let the demonstration run for 2–3 minutes. Observe and record all changes as the current flows through the electrolysis
solution. Be specific—compare the changes at the anode and the cathode. A yellow-brown suspension will form at the
anode. The solution around the cathode will turn dark pink and there will be rapid gas bubbling at the cathode. Discuss the
pH change responsible for the color change of the phenolphthalein indicator and the possible identity of the yellow-brown
product. Compare and contrast the reactions occurring at each electrode in the electrolysis of water (sodium sulfate) and
potassium iodide.
15. Remove the pencil lead electrodes from the electrolysis solution and disconnect them from the battery cap. Carefully rinse
the pencil leads with distilled water from a wash bottle and gently pat dry on a paper towel.
16. Add two drops of starch solution to the potassium iodide solution in the Petri dish and record observations in the data
table. The solution around the anode will turn dark blue or black (positive test for iodine).

Petri Dish Electrolysis continued
3
© 2016 Flinn Scientific, Inc. All Rights Reserved.
17. Pour the solution from the Petri dish into a waste beaker and rinse well with distilled water.
18. Place the clean Petri dish on the overhead projector stage and add about 20 mL of 1 M tin(II) chloride solution.
19. Connect one paper clip to each alligator clip lead on the battery cap and attach the paper clips to opposite sides of the Petri
dish.
20. Attach the battery cap with alligator clip leads to the 9-V battery and observe the changes at the anode and the cathode as
electrolysis proceeds. A milky-white cloudiness appears at the anode due to tin(IV) chloride and beautiful metallic tin(0)
crystals form at the cathode.
21. Allow the reaction to continue for 1–2 minutes and observe the pattern of crystal growth. Tin crystals grow in a feather-like
(fractal) pattern, producing a beautiful “crystal tree.”
22. Gently remove the alligator clip leads from the paper clips and carefully switch the polarity of the electrodes. Attach the
alligator clip from the negative battery post to the previous anode (left-hand side above) and the alligator clip from the
positive battery post the previous cathode (the right-hand side above.)
23. Observe the changes at the new anode and the new cathode. The reactions are reversed—new tin crystals grow from the
previous anode and the tin crystals at the previous cathode dissolve into solution.
24. Disconnect the battery cap from the battery to stop the reaction.
25. Carefully decant the solution from the Petri dish into a clean waste beaker (avoid pouring the tin crystals). The tin crystals
may be poured onto several layers of paper towels.
26. The electrolysis products may include dilute halogen solutions (chlorine, bromine, and iodine). Working in the hood,
carefully pour the contents of the Petri dish into a waste beaker containing sodium thiosulfate solution. Sodium thiosulfate
will reduce the halogen waste products. Allow the beaker to stand in the hood overnight.
Disposal
Please consult your current Flinn Scientific Catalog/Reference Manual for general guidelines and specific procedures govern-
ing the disposal of laboratory waste. Electrolysis of potassium iodide generates iodine. The waste solution may reduced with
sodium thiosulfate according to Flinn Suggested Disposal Method #12a. The acidic tin chloride waste solution may be neutral-
ized with base according to Flinn Suggested Disposal Method #24b.
Tips
• Electrolysis of sodium chloride generates chlorine gas at the anode. Based on their standard reduction potentials,
oxidation of chloride ion to chlorine (E
o
= –1.36 v) should be less favorable than oxidation of water to oxygen
(E
o
= –1.23 V). However, there is a significant overvoltage for oxidation of water, and oxidation of chloride competes
with oxidation of water under typical electrolysis conditions. Although the cause of the overvoltage is poorly understood,
it is generally believed to be due to a kinetically slow reaction at the anode.
• Potassium iodide solution is light- and air-sensitive. Prepare the solution fresh within two week of its anticipated use and
store the solution in a dark bottle.
• Electrolysis of salt solutions takes place at concentrations greater than about 0.2 M. We recommend using 0.5 M
solutions for potassium iodide and sodium sulfate—electrolysis is very rapid and the color changes are more obvious than
in 0.2 M solutions.
• A black coating forms on the paper clip electrodes when the paper clips are placed in the tin(II) chloride solution. This is
due to a spontaneous single replacement reaction between the metal in the paper clips and tin(II) ions. The black coating
will not interfere with the electrolysis reaction.
• Electrolysis is a challenging topic for students. The demonstration provides a great critical-thinking (inductive
reasoning) exercise for students to identify the products of the separate half-reactions based on the observations at each
electrode. Write down all of the possible oxidation and reduction half-reactions for each salt solution on the board, and
ask students to interpret the evidence and determine the actual products.

Petri Dish Electrolysis continued
4
© 2016 Flinn Scientific, Inc. All Rights Reserved.
• Have individual student groups research and then present a class seminar on (a) the historical role of electrolysis in the
discovery of potassium, sodium, magnesium, calcium, strontium, and barium; and (b) the modern importance of
electrolysis in the production of industrial chemicals, including aluminum, sodium hydroxide, chlorine, etc.
Discussion
An electrolytic cell consists of a source of direct electrical current connected to two electrodes in a solution of an electrolyte
or in a molten salt solution. The electrodes act as external conductors and provide surfaces at which electron transfer will take
place. Electrons flow from the anode, which is the site of oxidation, to the cathode, which is the site of reduction. The battery
or other voltage source serves as an electron “pump,” pushing electrons into the electrode from the negative pole and pulling
electrons from the electrochemical cell at the positive pole. The negative electrode, where the electrons enter the electrolysis
setup, is the cathode. The electrons are “consumed” in a reduction half-reaction. Electrons are generated at the anode, the
positive electrode, via an oxidation half-reaction. The migration of ions in the electrolyte solution completes the electrical
circuit.
The following half-reactions occur in the electrolysis of water.
Oxidation half-reaction (anode) 2H
2
O(l) → O
2
(g) + 4H
+
(aq) + 4e
–
Reduction-half-reaction (cathode) 2H
2
O(l) + 2e
–
→ H
2
(g) + 2OH
–
(aq)
Electrolysis of an aqueous salt solution may generate products other than oxygen or hydrogen if the salt contains ions that are
more easily oxidized or reduced than water molecules.
The electrolysis of aqueous potassium iodide (KI), for example, generates iodine at the anode and hydrogen gas at the cathode.
The products of the reaction demonstrate that oxidation of iodide ions (I
–
) to iodine (I
2
) occurs more readily than oxidation of
water. The overall reaction is the sum of the oxidation and reduction half-reactions.
Oxidation half-reaction (anode) 2I
–
(aq) → I
2
(s) + 2e
–
Reduction-half-reaction (cathode) 2H
2
O(l) + 2e
–
→ H
2
(g) + 2OH
–
(aq)
Overall reaction 2H
2
O(l) + 2I
–
→ H
2
(g) + I
2
(l) + 2OH
–
(aq)
The electrolysis of tin(II) chloride is an interesting example because tin(II) ions can be both oxidized and reduced. Tin(II) ions
are oxidized to tin(IV) ions at the anode and are reduced to metallic tin at the cathode. Insoluble tin(IV) chloride is observed as
a milky white precipitate at the anode.
Oxidation half-reaction (anode) Sn
2+
(aq) → Sn
4+
(aq) + 2e
–
Reduction half-reaction (cathode) Sn
2+
(aq) + 2e
–
→ Sn(s)
Overall reaction 2Sn
2+
(aq) → Sn
4+
(aq) + Sn(s)
Connecting to the National Standards
This laboratory activity relates to the following National Science Education Standards (1996):
Unifying Concepts and Processes: Grades K–12
Systems, order, and organization
Evidence, models, and explanation
Content Standards: Grades 9–12
Content Standard A: Science as Inquiry
Content Standard B: Physical Science, structure and properties of matter, chemical reactions, interactions of energy
and matter
Petri Dish Electrolysis continued
5
© 2016 Flinn Scientific, Inc. All Rights Reserved.
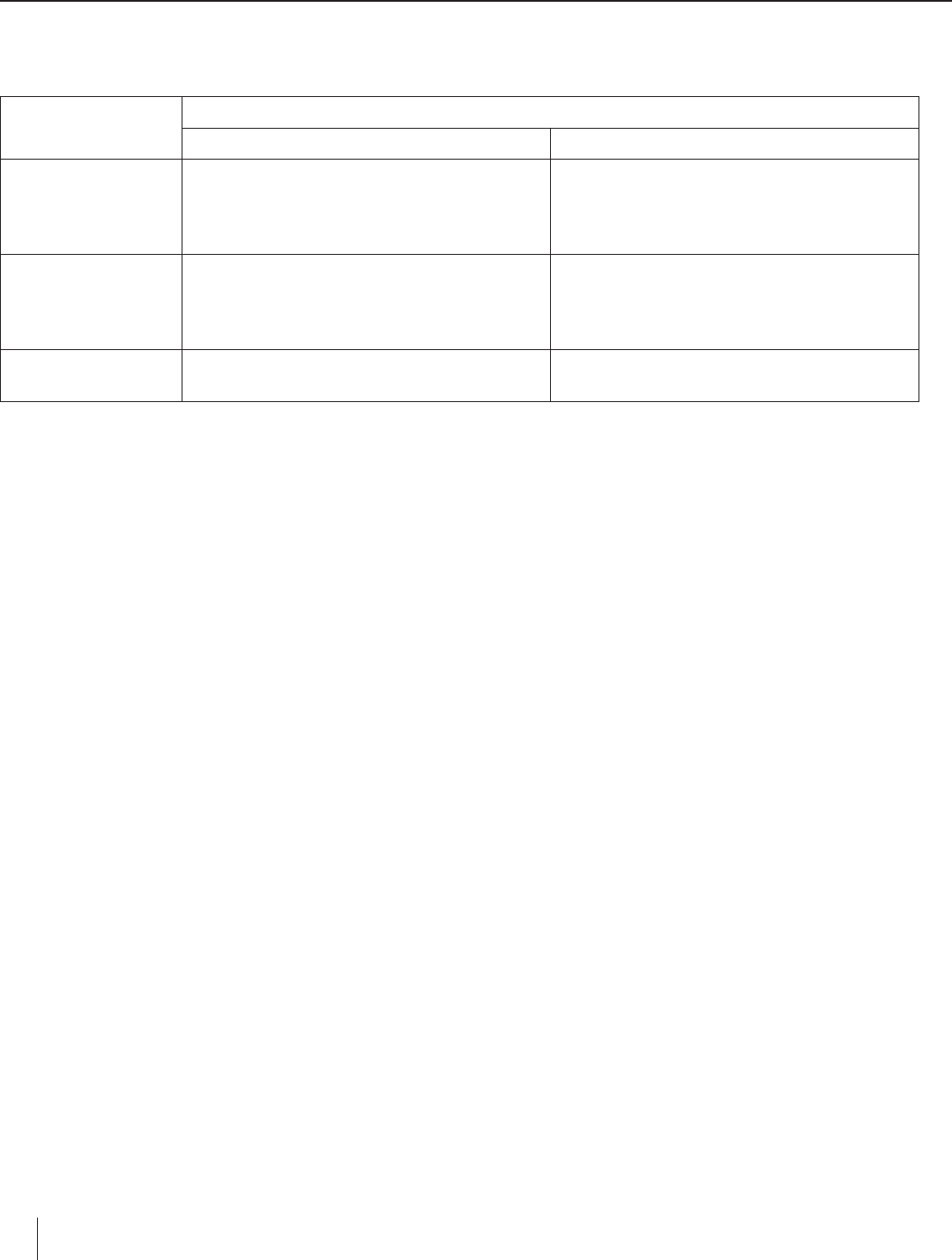
Answers to Worksheet
Data Table
Electrolyte
(Salt Solution)
Observations
Anode Cathode
Sodium Sulfate
Solution around the electrode gradually
turns yellow and then orange or red. Slow
gas bubbling—large bubbles appear to dis-
solve in solution.
Solution around the electrode quickly turns
purple and there is immediate and very rapid
gas bubbling. Lots of tiny little bubbles
appear to “pop.”
Potassium Iodide
Yellow substance formed at positive elec-
trode and dissolved in solution. Brownish-
yellow solid observed on electrode. Solution
turned black when starch was added.
Rapid gas bubbling observed at negative
electrode. Solution immediately surrounding
the cathode turned bright pink.
Tin(II) Chloride
Small amount of milky white precipitate or
cloudiness.
Beauiful metallic silver crystals grow in a
dendritic pattern, producing a “crystal tree.”
Answers to Discussion Questions
1. (a) Compare the color changes observed at the positive (+) and negative (–) electrodes in the electrolysis of sodium sulfate.
What ions were produced at each electrode?
The indicator color changed to red-orange at the (+) electrode. This is due to the formation of H
+
(H
3
O
+
) ions—universal
indicator is red in acidic solutions (pH <4), when the concentration of H
+
ions is greater than the concentration of OH
–
ions.
The indicator color changed to purple at the negative electrode. This is due to the formation of OH
–
ions—universal indicator is
purple in basic solutions (pH >10), when the concentration of OH
–
ions is greater than the concentration of H
+
ions.
(b) Write out the oxidation and reduction half-reactions for the decomposition of water and identify which reaction
occurred at each electrode, based on the indicator color changes.
Oxidation 2H
2
O(l) → O
2
(g) + 4H
+
(aq) + 4e
–
Reduction H
2
O(l) → H
2
(g) + OH
–
(aq)
2. Compare the rates of gas evolution at the positive (+) and negative (–) electrodes. What gas was produced at each electrode?
Explain, based on the balanced chemical equation for the decomposition of water.
The rate of gas evolution was greater at the negative electrode, where hydrogen gas was formed. According to the balanced chemical
equation for the decomposition of water, two moles of hydrogen gas are formed for every mole of oxygen gas that is released.
3. The following oxidation and reduction half-reactions are possible for the electrolysis of potassium iodide solution
(the solution components are water molecules, potassium ions (K+), and iodide ions (I–).
2H
2
O(l) → O
2
(g) + 4H
+
(aq) + 4e
–
2H
2
O(l) + 2e
–
→ H
2
(g) + 2OH
–
(aq)
K
+
(aq) + e
–
→ K(s) 2I
–
(aq) → I
2
(s) + 2e
–
(a) What product was formed at the anode in the electrolysis of potassium iodide solution? Explain based on your
observations—be specific!
The substance formed at the anode is an oxidation product. The product is yellow, water-soluble, and turns black when starch is
added—iodine.
(b) What product was formed at the cathode in the electrolysis of potassium iodide solution? Explain based on your
observations.
The substance formed at the cathode is a reduction product. The product is a gas, and is accompanied by the formation of a base
(phenolphthalein turned pink). The product is hydrogen, and hydroxide ions are formed as a byproduct.

Petri Dish Electrolysis continued
6
© 2016 Flinn Scientific, Inc. All Rights Reserved.
(c) Write the net ionic equation for the overall redox reaction in the electrolysis of aqueous potassium iodide.
Hint: Remember to balance the electrons!
2H
2
O(l) + 2I
–
→ H
2
(g) + I
2
(aq) + 2OH
–
(aq)
Flinn Scientific—Teaching Chemistry
™
eLearning Video Series
A video of the Petri Dish Electrolysis activity, presented by Irene Cesa, is available in Electrolysis Reactions, part of the Flinn
Scientific—Teaching Chemistry eLearning Video Series.
Materials for Petri Dish Electrolysis are available from Flinn Scientific, Inc.
Materials required to perform this activity are available in the Colorful Electrolysis and Electrolysis Reaction Kits available from
Flinn Scientific. Materials may also be purchased separately.
Catalog No. Description
AP6894 Electrolysis Reactions—Student Laboratory Kit
AP6467 Colorful Electrolysis—Chemical Demonstration Kit
P0019 Phenolphthalein Indicator Solution, 1%, 100 mL
P0171 Potassium Iodide Solution,0.5 M, 500 mL
S0353 Sodium Sulfate Solution, 0.5 M, 500 mL
S0151 Starch Solution, 0.5%, 500 mL
S0354 Tin(II) Chloride Solution, 1 M, 500 mL
U0001 Universal Indicator Solution, 100 mL
AP8954 Battery Clip with Alligator Clip Leads
GP3019 Petri Dish, Borosilicate Glass, 100 × 15 mm
AP1817 Pencil Leads
Consult your Flinn Scientific Catalog/Reference Manual for current prices.
Petri Dish Electrolysis continued
7
© 2016 Flinn Scientific, Inc. All Rights Reserved.
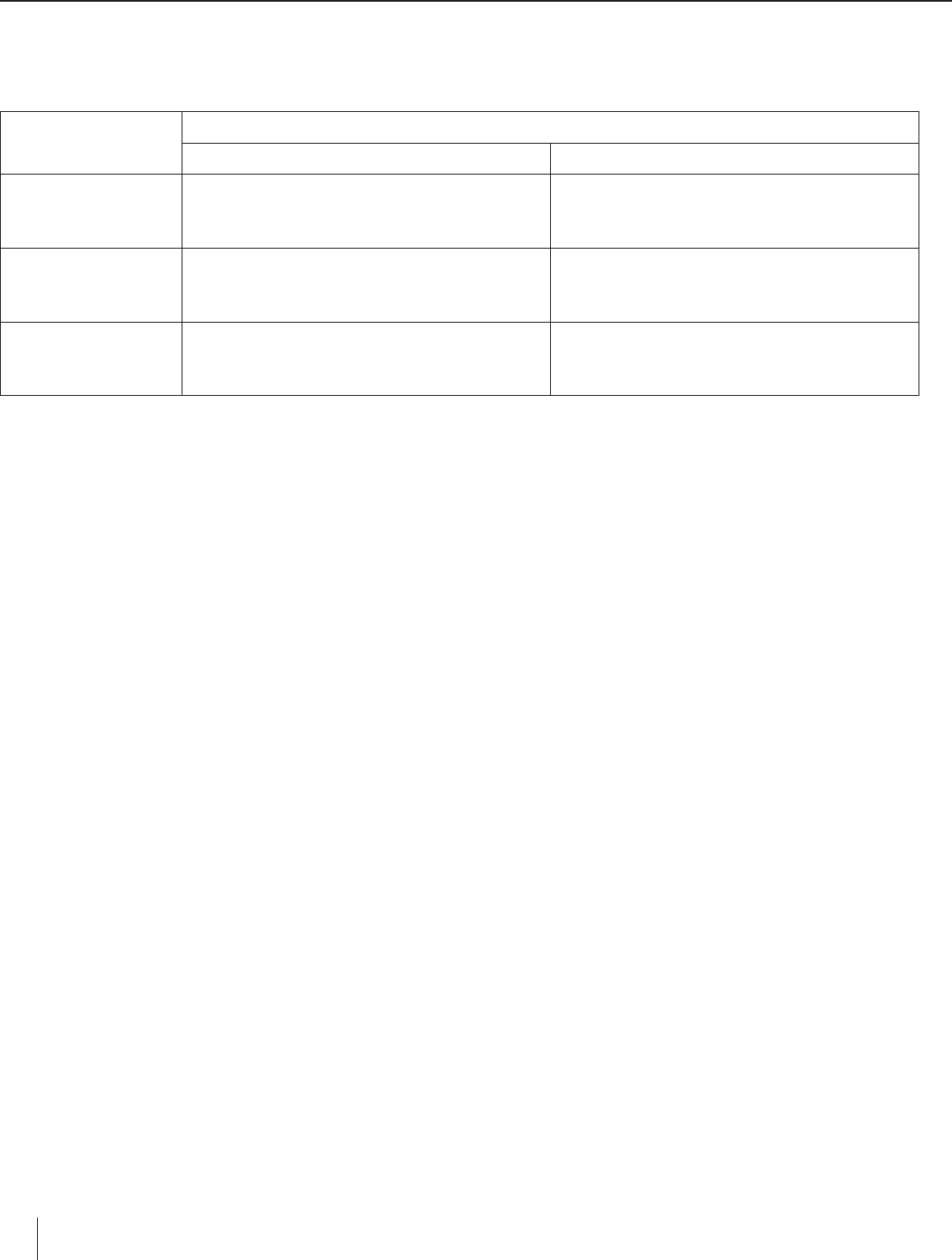
Petri Dish Electrolysis Worksheet
Data Table
Electrolyte
(Salt Solution)
Observations
Anode Cathode
Sodium Sulfate
Potassium Iodide
Tin(II) Chloride
Discussion Questions
1. (a) Compare the color changes observed at the positive (+) and negative (–) electrodes in the electrolysis of sodium sulfate.
What ions were produced at each electrode?
(b) Write out the oxidation and reduction half-reactions for the decomposition of water and identify which reaction
occurred at each electrode, based on the indicator color changes.
2. Compare the rates of gas evolution at the positive (+) and negative (–) electrodes. What gas was produced at each electrode?
Explain, based on the balanced chemical equation for the decomposition of water.
3. The following oxidation and reduction half-reactions are possible for the electrolysis of potassium iodide solution
(the solution components are water molecules, potassium ions (K
+
), and iodide ions (I
–
).
2H
2
O(l) → O
2
(g) + 4H
+
(aq) + 4e
–
2H
2
O(l) + 2e
–
→ H
2
(g) + 2OH
–
(aq)
K
+
(aq) + e
–
→ K(s) 2I
–
(aq) → I
2
(s) + 2e
–
(a) What product was formed at the anode in the electrolysis of potassium iodide solution? Explain based on your
observations—be specific!
(b) What product was formed at the cathode in the electrolysis of potassium iodide solution? Explain based on your
observations.
(c) Write the net ionic equation for the overall redox reaction in the electrolysis of aqueous potassium iodide.
Hint: Remember to balance the electrons!
