
Borax Crystal Ornaments
Introduction
Add some sparkle to your next holiday celebration by making ornaments of borax crystals.
Simply dissolve sodium borate in hot water, suspend a shaped chenille wire into the solution
and wait. The next day remove a beautiful crystal-covered ornament from the solution!
Concepts
• Crystal formation • Solutions • Solubility
Materials
Sodium borate, Na
2
B
4
O
7
z10H
2
O, 50 g Ruler
Water, distilled or deionized (DI) Scissors
Balance, 1-g precision Scoop or spoon
Beaker, 400-mL, borosilicate glass Stereoscope or magnifying lens
Beaker tongs Stirring rod
Chenille wire String
Heat-resistant surface Thermometer
Hot plate Weighing dish
Paper towel Wood splint or pencil
Safety Precautions
Sodium borate may be harmful if swallowed and high doses may damage fertility or the unborn child. Use caution when handling hot
glassware. Use beaker tongs or wear heat-resistant gloves. Handle the chenille wires carefully as the ends of the wires may be sharp.
Wear chemical splash goggles, chemical-resistant gloves and a chemical-resistant apron. Please review current Safety Data Sheets for
additional safety. handling and disposal information. Wash hands thoroughly with soap and water before leaving the laboratory. Please
follow all laboratory safety guidelines.
Procedure
1. Obtain one chenille wire. Bend the wire into desired ornament shape. Note: Chenille wires may be cut with scissors to
shorter lengths. Be careful when bending or twisting the wire as the ends are sharp.
2. Cut a 9″ piece of string and tie it to the shaped wire so the ornament will hang in balance.
3. Holding the string, lower the ornament into an empty 400-mL beaker to make sure the ornament will fit completely
in the beaker without touching the sides or bottom. If the ornament does not fit, adjust the shape to make it smaller.
Remove the ornament from the beaker and set aside for step 10. Note: Two smaller ornaments may fit into a 400-mL
beaker if they do not touch each other or any part of the beaker.
4. Add 300 mL of distilled or deionized water to the beaker and place on a hot plate.
5. Heat the water to just below boiling, at least 80 °C.
6. While the water is heating, place a weighing dish on a balance and tare the balance.
7. Weigh 50 g of sodium borate in the weighing dish and then set aside for step 9.
8. When the water temperature has reached 80–85 °C, use beaker tongs to carefully remove the beaker from the hot plate
and place it on a heat-resistant surface. Turn off the hot plate.
9. Carefully add the 50 g of sodium borate to the hot water and stir with a stirring rod until dissolved.
10. Holding the ornament by the end of the string, lower it into the sodium borate solution until the ornament is com-
pletely covered by the solution but is not touching the bottom or sides of the beaker.
© 2019 Flinn Scientific, Inc. All Rights Reserved. 1
Publication No. 11600
103119
SCIENTIFIC CANADA
Borax Crystal Ornaments continued
2
© 2019 Flinn Scientific, Inc. All Rights Reserved.
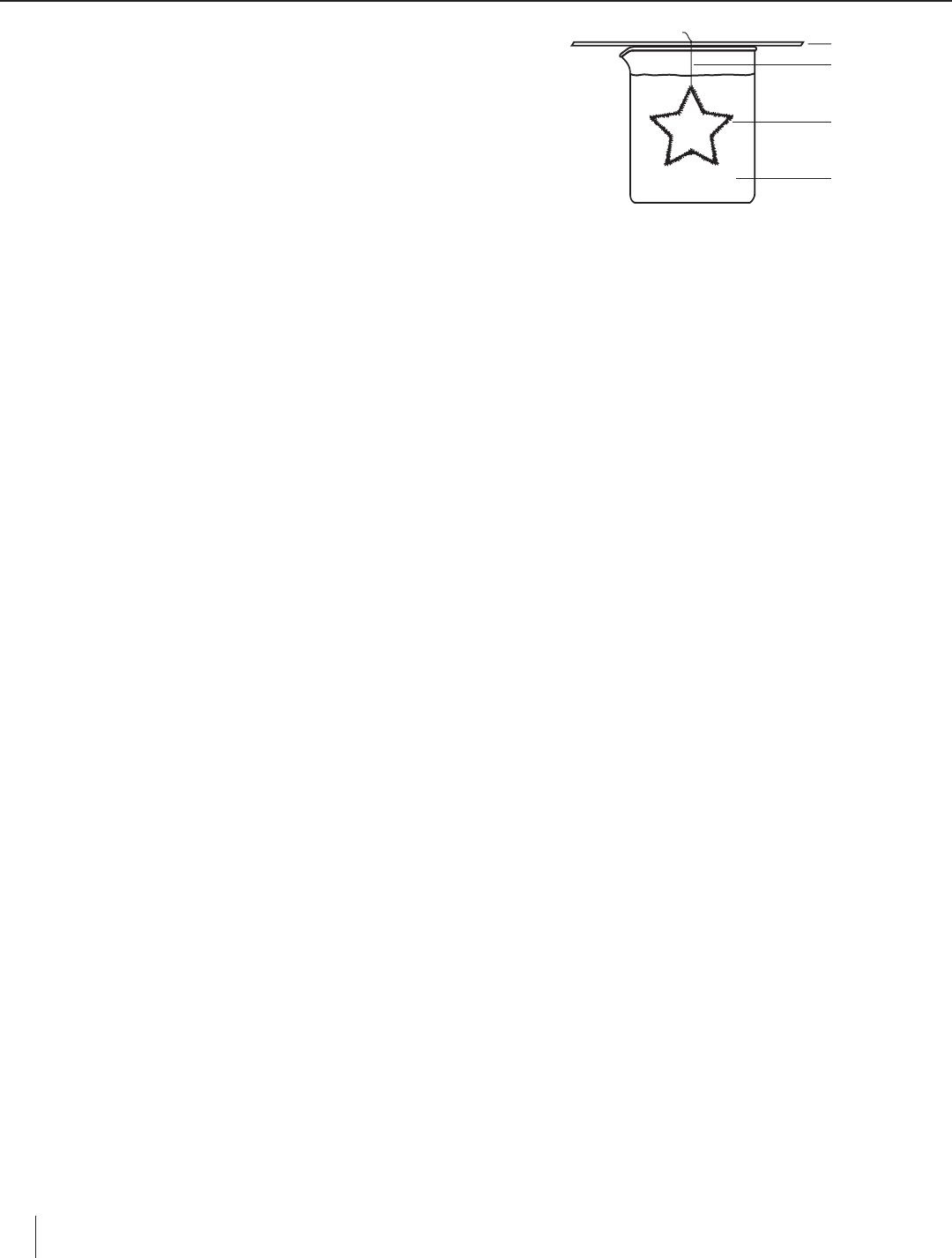
11. Wrap the excess string around a wood splint or pencil and place the
splint across the top of the beaker to keep the ornament suspended in
the solution (see Figure 1).
12. Allow the solution to cool slowly overnight and the crystals to “grow”
on the ornament.
13. The next day, carefully remove the ornament from the beaker and
place on a folded paper towel to dry.
14. Look at the crystals with a strong magnifying lens or stereoscope and
try to identify the shape.
Disposal
It is recommended that you consult your local school board and/or municipal regulations for proper disposal methods that may
apply before proceeding.
Tips
• This is a wonderful integrated activity that combines science and art and may be used as a science activity for any holiday
or as a component for a unit on crystallization, solutions and solubility or even mineral crystal shapes.
• Use the crystal ornaments to decorate your classroom or lab. It is not recommended to allow students to take chemicals
home.
• If time permits, turn this into an inquiry activity. Allow students to design and carry out an experiment to test the
variables of concentration or temperature of the sodium borate solution and observe the effects on the formation of the
crystals.
• Students may also make ornaments with alum crystals (aluminum potassium sulfate). Follow the same procedure as with
the borax crystal ornaments, but after step 3, use a small paintbrush to brush a small amount of white glue on the surface
of the ornament. Before the glue dries, sprinkle the ornament with 10–15 g of alum. Allow the ornament to dry. Use
100 g of alum instead of 50 g of sodium borate in steps 7 and 9. Alum crystals will appear cubic or octahedral, depending
upon the growing conditions.
• Have students look at salt (sodium chloride) crystals under magnification to compare their cubic shape to the monoclinic
sodium borate crystals.
• If enough hot plates for a class are not available, an option for hot water is to use a large hot pot or coffee percolator.
Dispense the hot water into the beaker just prior to adding the pre-measured sodium borate. A laboratory microwave is
another option. Be sure to use beaker tongs or wear heat-resistant gloves when handling glassware filled with hot water.
• Borosilicate glass beakers or canning jars of varying sizes may be used. Adjust the amount of water and sodium borate
accordingly, keeping a 1:6 ratio of sodium borate to water. Lab partners may share a large beaker.
• The sodium borate crystals will not take up food dye and therefore colouring the solution will have no effect. The colour
of the chenille wire will determine the colour of the ornament. You may wish to provide several different coloured wires
for students to cut and combine to expand the creative possibilities.
• If crystals have grown on the bottom or sides of the beaker, simply reheat the solution to dissolve the borax before
disposal.
Wood splint
String
Chenile wire
ornament
Sodium borate
solution
Figure 1.
Borax Crystal Ornaments continued
3
© 2019 Flinn Scientific, Inc. All Rights Reserved.
Discussion
Solutions and Solubility
A solution is a mixture of two or more pure substances that is homogeneous or uniform throughout. The substance that is being
dissolved is called the solute, and the substance that does the dissolving is called the solvent. Solubility is the amount of solute that
will dissolve in a given amount of solvent at a particular temperature. Solubility generally increases with increasing tempera-
ture when dissolving solids into liquids. Solubility also depends on the substance being dissolved. Some salts are very soluble in
water, while others are only slightly soluble.
A solution is said to be unsaturated if its solute concentration is less than its solubility. When a solute’s concentration is equal to
its solubility, the solution is said to be saturated. At that temperature, no more solid can be dissolved in the solution. However,
if a saturated solution is heated, the solubility of the solute may increase, making it possible to dissolve more solid in that same
solution. If additional solid is added to the hot solution and then cooled, often the extra solid will precipitate or crystallizes out
of solution.
Unit Cells
The macroscopic regularity in the shapes of ice crystals, snowflakes, crys-
talline salts and gemstones suggests that crystals must possess some sort of
atomic-level regularity. This regularity is called a crystal lattice, and every
crystal is built upon one. A crystal lattice is a repeating, orderly arrangement
of atoms, molecules, and ions. The specific repeating pattern unique to each
crystal lattice is called a unit cell, the smallest repeating pattern that reflects
the macroscopic shape of the crystal. Sodium borate is an ionic compound.
Sodium and borate ions are arranged into a regular three-dimensional pat-
tern resulting from a net balance of attractive and repulsive forces. This
arrangement forms an extended network, constructed by repeating the
unit cell pattern over and over again in all three dimensions and the crystal
“grows.”
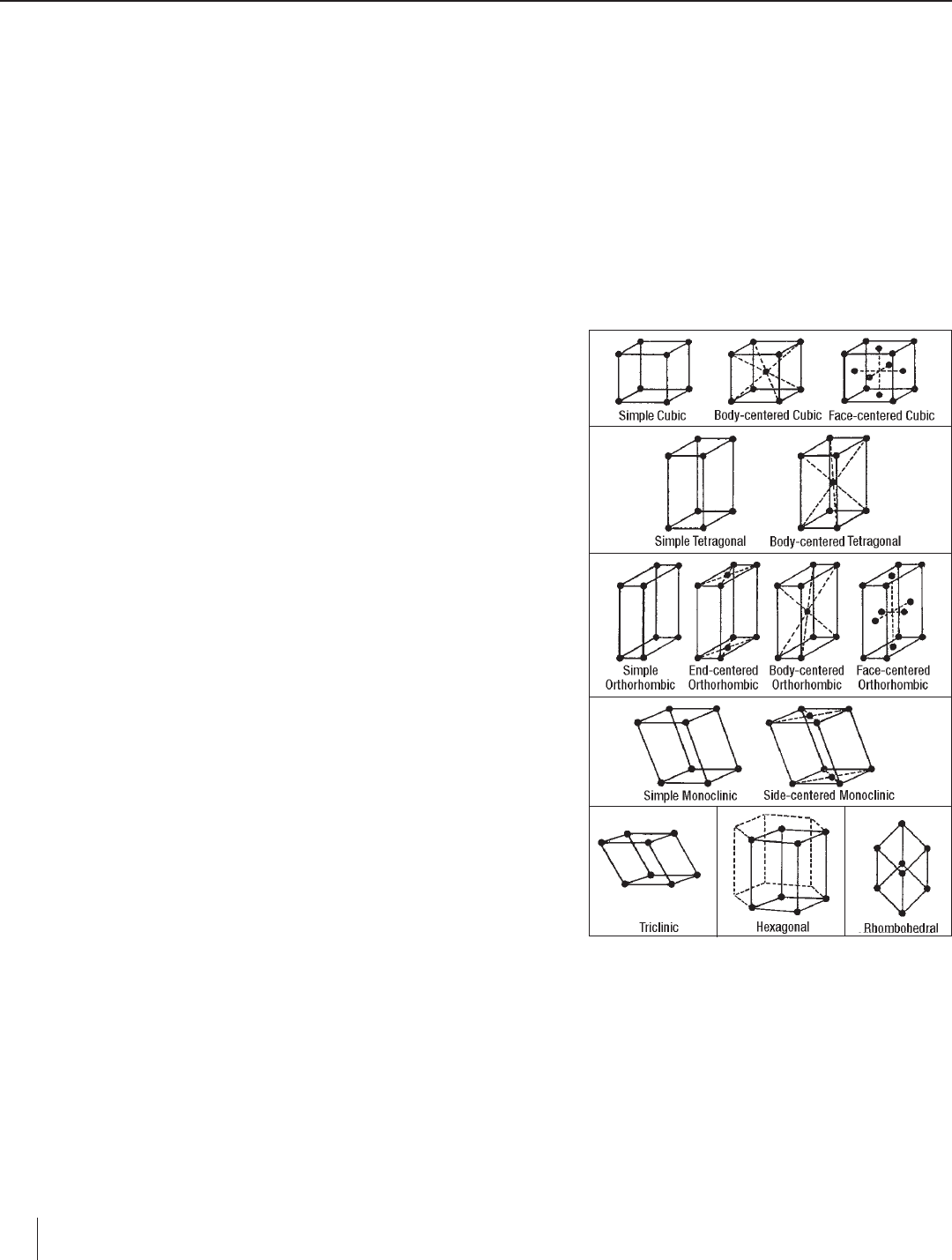
Seven types of unit cells occur in nature—cubic, tetragonal, ortho rhombic,
monoclinic, triclinic, hexagonal and rhombohedral. Several of these types
of unit cells have variations. The base unit cell plus its variations make up
the unit cells for a given crystal system. The seven types of unit cells, their
variations and associated crystal structures are illustrated in Figure 2.
Although the unit cell for a particular solid will always be uniform, varia-
tions in crystal shapes occur because the growing solution’s concentration
varies from one point to another around the crystal. If a particular face of
the crystal is surrounded by solution that is more concentrated, it will grow
faster than other faces, which are surrounded by less concentrated solution.
In addition, the different types of faces have different inherent growth rates.
The specific shape of the crystal that forms is determined by the rates at
which its various faces grow.
Other factors also affect crystal growth. One of the most important factors
is the temperature at which crystals are grown. A constant temperature is
very important for growing large crystals. If the temperature varies dur-
ing crystal growth, the solubility of the solute changes. If the solubility
increases, then the crystals may begin to dissolve since the solvent can now
accept more solute in solution. Another factor affecting the quality and size of crystals is the rate at which they are grown. Slow
growth results in larger quality crystals. If crystals are grown too fast—for example, if the solutions are cooled too quickly after
heating—the crystals will be smaller and cloudy in appearance. Crystals also need room to grow and may be smaller or overlap
if there is a limited area for growth.
Figure 2. The seven types of unit cells—cubic,
tetragonal, orthorhombic, monoclinic, triclinic,
hexagonal, and rhombohedral

Borax Crystal Ornaments continued
4
© 2017 Flinn Scientific, Inc. All Rights Reserved.
Reference
Borax Snow Crystals, Indiana Alliance of Chemistry Teachers, 2009 HASTI Presentations, http://www.chem.purdue.edu/iact/
HASTI.htm (accessed September 2016).
Materials for Borax Crystal Ornaments are available from Flinn Scientific Canada.
Catalogue No. Description
APJ7413 Crystal Ornaments—Holiday Laboratory Kit
SJ0435 Sodium Borate, 500 g
AP8910 Chenille Wires, 1-ft, 10/pkg, White
AP8859 Chenille Wires, 1-ft, 10/pkg, Red
AP8860 Chenille Wires, 1-ft, 10/pkg, Blue
AP8861 Chenille Wires, 1-ft, 10/pkg, Green
AP8862 Chenille Wires, 1-ft, 10/pkg, Black
GP1025 Beaker, Borosilicate Glass, 400-mL
AP1240 Ceramic Fiber Squares, 4″ × 4″, 12/pkg
AB1134 Dual Lens Magnifier
AP9807 Flinn Digital Hot Plate, 7" × 7"
Consult your Flinn Scientific Catalogue/Reference Manual for current prices.
